Clinical Instrumentation
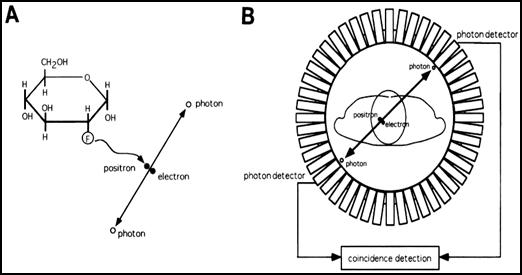
Figure 2 shows the detection scheme of the positron camera. (A) After its origin, the positron emitted from the Fluorine-18, is annihilated with a negative electron, giving rise to two photons of 511 keV range each, leaving in opposite directions almost 180 ° which are detected in coincidence by the crystal ring PET camera (B). This allows their exact location and make it possible the reconstruction of tomographic images.

PET systems are composed of a set of rings with block detectors with
bismuth germanate crystals (BGO)
or Lutetium Oxyorthosilicate
crystals (LSO), coupled to photomultipliers.
The BGO crystals
are denser than the INa (Tl) and therefore more efficient to detect the
511
keV photons. On the other hand
they are not hygroscopic and can therefore
be cut into
parallelepipeds a few millimeters thick (e.g.
4 or 5 mm), the magnitude of spatial
resolution. PET systems are also built with crystals of INa (Tl), which are cheaper,
and
despite being less efficient detecting the 511 ke photons, are used
for
clinical PET. In favor of
the INa (Tl) is the fact that
emits a higher percentage of light and the
signal in the crystal decays faster,
therefore better resolution and lower dead time,
though has less efficiency than the BGO crystal.
PET
coincident detection between
crystals of the same ring
allows a two dimension (2D)
reconstruction.
Lead septa
are installed to separate the rings
and avoid
random coincidences and scattered among non-contiguous planes.
The latest generation of
PET systems may do acqusitions
without septa employing 3D
reconstruction algorithms. With this
technique, the coincidence
detected events are
possible not only with crystals of
same rings
but also between rings, increasing
sensitivity. One of the
big advantages
of PET is its ability to correct for attenuation of radiation in the
tissues
by the method of transmission using 68Ge or 137Cs sources
ot the X-rays used in the CT.
The ratio of the absorption
with and without the patient
is used to
calculate the absorption
coefficient
(m)
for each projection. Once attenuation
and
scattered radiation have been
taken care of,
activity within the tissues of the patient can be quantified in absolute
terms.
For example,
to
obtain quantitative images of FDG with plasma data
included
into kinetic models from which to calculate glucose uptake into tissues.
In addition, PET images can be quantified to determine the degree of radiotracer
uptake, using the SUV (Standardized Uptake Value) index . This relates tumor metabolic activity
compared to the injected dose and patient weight. As a cutoff point of
reference a value = 2.5 may be helpful. Benign lesions tend to
be below and malignant
abnormalities
frequently over this value, but
this only is
helpful as a general orientation. Even more useful, is for follow-up. In the
case of a good therapeutic response, the value
should
drop significantly. The
patient is his own control.
Page 8 Home Index Clinical Nuclear Medicine Therapeutic Methods Previous Page Next Page