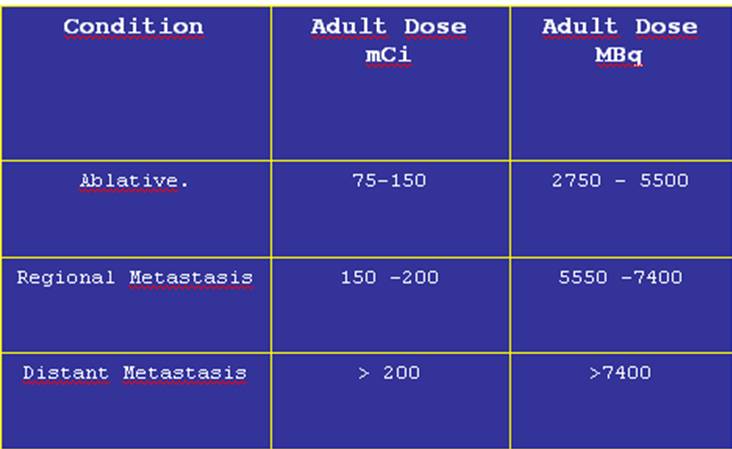
131I Differentiated Thyroid Cancer Therapy Dose Estimation
The table below shows frequently accepted fixed doses according to the tumor extension. Other authors recommend the use of dosimetric methods to calculate the therapeutic dose more exactly, but usually this procedure is more cumbersome and not practical.

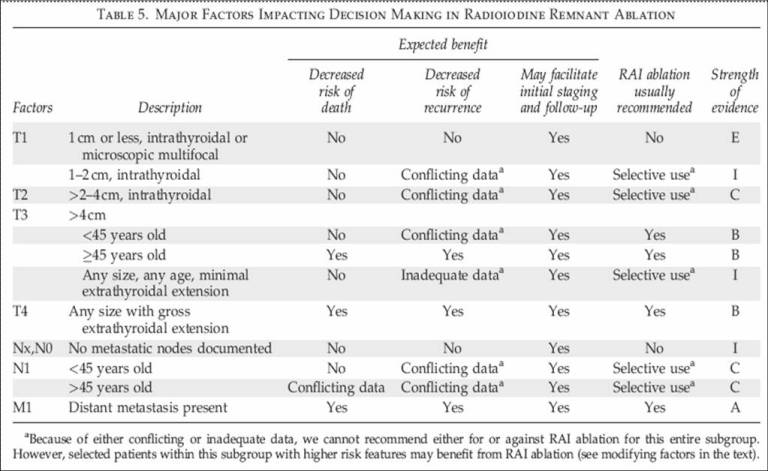
In ablative therapy there is stil some controversia regarding which patients
could benefit from the procedure. In relation to this topic, it is of interest
to reproduce the criteria suggested by The American Thyroid Association
Guidelines Taskforce and summarized in the following table.
It is important to consider risk factors to make a decision in the individual
patient. Patient age > 45 y of greater risk compared to less than 45 y.
Histology, follicular and Hurthle cell cancer are of higher risk compared to
papillary cancer. Size, those > 1 cm compared to less than 1 cm. Capsular and
vascular invasion. Tumor extrathyroidal extension. Lymphnode involvement.
Distant metastasis (2).
In two recent articles, the suggestion in the use of a low dose of radioiodine
and recombinant TSH to treat low risk cancer patients would be as effective as a
larger dose and the withdrawal of thyroid hormones (5,6). Their end point was
the efficiency in ablation reaching 90% at 9 months. Follow up was less than a
year. These are a couple of interesting papers, but the information reported
needs to be confirmed in other researchs and including recurrence incidence and
long term follow up.
Author Experience and Recommendation:
Almost every patient today qualifies for the ablative therapy regardless of the
presence of risk factors, taking the advantage that the ablation will allow
accurate staging and to get more specific results from thyroglobulin and whole
body scans, when remnant thyroid tissue is completely eliminated, again see
table below in expected benefit. In about 83% of cases a 100 mCi dose of 131-I
will destroy all thyroid parenchyma and the rest will need a second treatment.
On the other hand, secondary effects of radioiodine are acceptable.
In children the dose can be adjusted by weight or body surface area. For instance, 1/3 in a 5 year old child, 1/2 at 10 years of age and 5/6 in a 15 year old youngster. Just as a reference radioiodine at 37.0 to 92.5 MBq/kg of body weight, may be administered.
The generally accepted absorbed dose thresholds providing high efficacy are ≥
300 Gy to remnants or ≥80 Gy to tumour deposits. The generally accepted
surrogate dose threshold to avoid serious myelotoxicity is a blood absorbed dose
≤2 Gy.
References:
1 Jairo Wagner, Anneliese F. Thom, Lilian Yuri Itaya Yamaga. Tratamiento del
Cancer Diferenciado del Tiroides con Radio Yodo, P: 193 -201 In: Medicina
Nuclear Aplicaciones Clínicas. Eds: I. Carrio - P. González. Editorial Masson,
Barcelona España, 2003.
2 Cooper David S. et al. Revised American Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer. THYROID 2009; 19: 1167-1214.
3 Jarzab B, Handkiewicz-Junak D, Wloch J. Juvenile differentiated thyroid carcinoma and the role of radioiodine in its treatment: a qualitative review. Endocr Relat Cancer. 2005;12 (4):773–803.
4 Luster M. et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008 Oct;35(10):1941-59.
5 Schlumberger M at al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012 May 3;366(18):1663-73.
6
Mallick U. et al. Ablation with low-dose radioiodine and thyrotropin alfa in
thyroid cancer. N Engl J Med. 2012 May 3;366(18):1674-85.