Lymphomas
Lymphoma FDG Avidity According to WHO
VALUE OF PET-CT FDG PROGNOSTICATION AFTER THERAPY IN LYMPHOMA
Introduction
PET prognosis value with the use of visual interpretation may be limited due to high false positive rate. In fact in some studies, active lymphoma in residual masses after 4 cycles of dose-dense R-CHOP was related to a biopsy-proven, in only 23 % of cases (1).
The key challenge is to set up reproducible interpretation criteria that are able to both identify FDG uptake associated with active disease and minimize the risk of interpreting overlapping FDG uptake related to nonspecific post-therapy inflammatory changes such as active lymphoma.
International Harmonization Project (IHP)
The International Harmonization Project (IHP) criteria were purely visual, and used either
the mediastinal blood pool as background reference for residual mass equal or greater than 2 cm
the surrounding background for mass less than 2 cm (2).
However, when the IHP criteria were strictly applied to evaluate interim PET after 2 or 4 cycles of immunochemotherapy, they were unable to identify patients with different outcomes, mainly due to a low (about 30 %) positive predictive value.
Deauville criteria
The Deauville criteria, designed as a semiquantitative visual analysis using a 5- point scale (5PS), were found to improve the accuracy of the interpretation compared to IHP criteria:
The residual mass uptake is then compared to the liver uptake, a 5PS score of four or five being required to consider a positive PET.
1. No uptake;
2. Uptake ≤ mediastinum;
3. Uptake > mediastinum but ≤ liver;
4. Uptake moderately increased compared to the liver at any site;
5. Uptake markedely increased compared to the liver at any site or/and new sites of disease (3).
The better discrimination of the liver background reference seems mainly related to a higher intensity of the liver FDG uptake (SUVmax toward 2.4), compared to the mediastinal blood pool reference background (SUVmax toward 2).
Thus, a residual mass with an FDG uptake higher than the liver background better differentiates from the background noise and has less risk to be attributed to a nonspecific uptake.
To establish its prognosis impact, an international validation study of 5PS was launched in 2010, enrolling 120 patients from 5 institutions in Europe and the United States, who were treated with an R-CHOP regimen with an available PET2 and no treatment change on the basis of PET results. A retrospective PET review was performed by three independent experts.
This study showed that PET2-negative patients have a significantly better 2- year event-free survival than PET2-positive patients on the basis of 5PS criteria (83 % vs 56 %; P<0.001).
ΔSUVmax (Semiquantitative Approach)
According to IHP criteria, patients with a positive PET2 who achieved an ΔSUVmax greater than 66 % and patients with a negative PET2 had a similar 2-year progression-free survival.
Similarly, patients with both a visually positive PET4 and an ΔSUVmax higher than 70 % and those with a negative PET4 had a consistent outcome. Interestingly, the 80 % of PET4-positive patients reclassified as good responders on the basis of ΔSUVmax in this series were consistent with the 87 % patients with positive PET4 who had no evidence of lymphoma after biopsy of the mass with residual FDG uptake in the study by Moskowitz et al (4).
This suggests that the semiquantitative approach helps to identify false-positive cases generated by the visual analysis and may avoid the biopsy of the residual lesion uptake in most cases. Thus, in the GELA series, the eight PET4-positive patients who achieved an ΔSUVmax greater than 70 % and underwent a biopsy of the residual hypermetabolic mass had no evidence of lymphoma (1).
PitfallS of the ΔSUVmax analysis
1) The main pitfall of the ΔSUVmax analysis is related to the absolute requirement of a baseline PET to allow an ΔSUVmax calculation. This could be a concern in high-risk DLBCL patients who need immediate treatment. However, even for a visual PET interpretation, it is advisable to perform a baseline PET to facilitate the evaluation of subsequent scans.
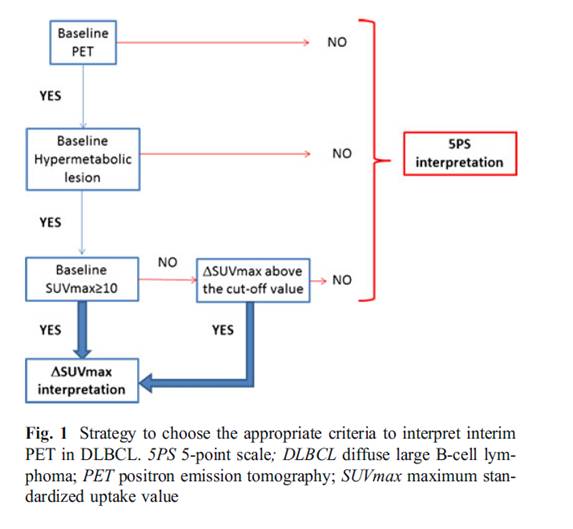
2) ΔSUVmax analysis can generate false-positive results when the baseline SUVmax is low and specifically less than 10. This occurred in two patients (2 %) of the LNH2007-3B interim analysis, leading to an ΔSUVmax lower than the defined cut-off value. Both cases were easily identified, since PET2 and PET4 were negative according to visual analysis.
3) In the rare cases in which no hypermetabolic lesion is detectable because of a complete resection of the tumor mass at diagnosis, the semiquantitative approach is obviously unsuitable.
4) In situations when the ΔSUVmax approach is not possible, the response has to be assessed according to 5PS criteria. In these cases, it has been proposed to use an SUV quantification of the liver to improve the positive predictive value and the reliability of the 5PS interpretation. A cut-off value of 140 % of the SUVmax liver might be more appropriate to differentiate lymphoma uptake from no specific uptake.

5) The need of the PET procedure standardization to allow the SUVmax calculation. Specifically, the weight of the patient at time of PET examination and the injected activity of 18F-FDG are critical.
References:
1 Rene-Olivier Casasnovas, Michel Meignan, Alina Berriolo-Riedinger, Emmanuel Itti, Damien Huglo, Corinne Haioun and Franck Morschhauser. Early Interim PET Scans in Diffuse Large B-Cell Lymphoma: Can There Be Consensus About Standardized Reporting, and Can PET Scans Guide Therapy Choices?. Curr Hematol Malig Rep (2012) 7:193–199.
2 Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the imaging subcommittee of international harmonization project in lymphoma. J Clin Oncol. 2007;25(5):571–8.
3 Meignan M, Itti E, Gallamini A, Haioun C. Interim 18Ffluorodeoxyglucose positron emission tomography in diffuse large B-cell lymphoma: qualitative or quantitative interpretation–where do we stand? Leuk Lymphoma. 2009;50(11):1753–6.
4 Moskowitz CH, Schöder H, Teruya-Feldstein J, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDGPET in advanced-stage diffuse large B-cell lymphoma. J Clin Oncol. 2010;28(11):1896–903.
Home Index Clinical Applications Hodgkin’s Non Hodgkin’s disease